Museums theme – Quest for Absolute Zero: A Human Story about Rivalry and Cold
Abstract
https://dx.doi.org/10.15180/170812/001In 2008 Rijksmuseum Boerhaave organised an exhibition on the occasion of the centennial of the liquefaction of helium by Kamerlingh Onnes in Leiden. Quest for Absolute Zero was the first major temporary exhibition aimed at new target groups. Therefore the focus was on the human scale and the colourful story behind the ‘man of Absolute Zero’. The fierce competition with James Dewar from the Royal Institution was also highlighted. The outcome of Quest for Absolute Zero was extremely positive for Rijksmuseum Boerhaave in terms of visitor numbers, media coverage, collaborations and side events. The project also proved to be a very useful lever for the renewal of the permanent exhibition, scheduled to open in December 2017.
Keywords
cold, exhibition, Kamerlingh Onnes, liquid helium, storylines, target groups
Introduction
https://dx.doi.org/10.15180/On 10 July 2008, at four o’clock in the afternoon, the opening celebrations were held at Rijksmuseum Boerhaave in Leiden for the exhibition The Quest for Absolute Zero: A Human Story about Rivalry & Cold. Earlier that afternoon, in the nearby Lakenhal, the art museum that tells the history of the city of Leiden, an accompanying exhibition was opened, entitled The Kamerlingh Onnes Family: Cold & Art. The invited guests processed from the Lakenhal to Rijksmuseum Boerhaave, between blue helium-filled balloons, each of which represented a progressively lower temperature, passing containers of deep-frozen ice ‘steaming’ with cold, and ending at the absolute zero point of -273 degrees Celsius at Rijksmuseum Boerhaave. On arrival, the welcoming drink took the form of a cocktail, which itself appeared to steam through the addition of a block of dry ice (frozen carbon dioxide) at the base of the glass. In the museum’s walled garden, performers juggled with ice creams, students performed cold tests and a chef prepared cryogenic snacks that went like ‘cold cakes’. All in all, it proved a hugely successful happening, and the ‘kick off’ to a heavily-visited twin exhibition that marks a turnaround in the history of Rijksmuseum Boerhaave and reflects the new vision of the museum on the presentation of science and technology.
The background to the exhibition was the centennial of the greatest success in the career of Heike Kamerlingh Onnes (1853–1926): the liquefaction of helium on 10 July 1908. On that wet, windy summer’s day, the physicist from Leiden outdid his arch rival James Dewar, director of the Royal Institution in London, after a long period of continuous hard work in his cryogenic laboratory (Onnes, 1909). His achievement was a technical marvel that opened up an entirely new field of fundamental research. With a temperature of less than two degrees above absolute zero, Leiden became the coldest place on Earth – a position it succeeded in maintaining until the 1950s.[1] The ‘victory over helium’ still stands as a milestone in the history of physics. Liquid helium laid the path to spectacular discoveries in extreme cold, such as superconductivity (Onnes, 1911), superfluidity (Kapitza, 1938), and Bose-Einstein condensation (Anderson et al, 1995).
Rijksmuseum Boerhaave
https://dx.doi.org/10.15180/170812/002It could hardly be described as a surprise that Rijksmuseum Boerhaave developed the plan of holding an exhibition to celebrate one hundred years of liquid helium. After all, the very roots of the museum lie in physics in Leiden. Leiden physics reached its zenith with the appointment of Heike Kamerlingh Onnes as a professor in experimental physics in 1882. With a will of iron, tremendous perseverance, amazing powers of conviction, incredible vision and courage, he set himself the task of experimentally testing and expanding on the molecular theories of his friend and mentor Johannes Diderik van der Waals, with the aim of harvesting international acclaim for Dutch physics. To be able to implement his programme, Kamerlingh Onnes transformed the solid but somewhat sleepy teaching institute of his predecessor Rijke into a bustling research laboratory of international allure (Delft, 2007).
As part of the operation, the collection of demonstration instruments of the Leiden Physics Cabinet, comprising lenses by Christiaan Huygens and the marvellous eighteenth-century demonstration appliances used by Jacob Willem’s Gravesande in his lectures (and with which he attracted students to Leiden from all across Europe) was mercilessly relegated to the attic rooms of the laboratory. Air pumps, levers, collision devices, centrifugal machines: in Kamerlingh Onnes’ world, they were above all obstacles. In 1887, a whole collection of lenses, the pendulum clock fitted with cycloid arcs, and a planetarium by Huygens were transferred to the Leiden Observatory, together with a number of old globes. In his annual report for 1887, Kamerlingh Onnes wrote, ‘It was with some sadness that I bade farewell to these objects that originated with our great compatriot Huygens’. The professor and director would have loved to have paraded them in his attic, he added, but ‘in the absence of support’ that was out of the question.
Claude August Crommelin, appointed curator of the Physics Laboratory in 1907, took to heart the fate of this attic collection. Crommelin, who arrived in Leiden in 1898 and attained his doctoral degree under Kamerlingh Onnes in 1910, was born into an aristocratic family. A music lover (he played piano and cello and together with Ehrenfest and Einstein performed in a trio, whenever the latter was staying in Leiden), he was a fervent museum visitor with a keen interest in the history of the natural sciences. On the occasion of the fortieth anniversary of Kamerlingh Onnes’ professorship at Leiden, he organised an exhibition in the Physics Laboratory with tables, drawings and graphs as well as original objects such as the magnet with which the Zeeman effect was discovered in 1896, and the fragile helium liquefier from 1908 (see Figure 1). Crommelin was fully aware of the cultural and historical value of these instruments, and guarded the well-being of the material heritage of his laboratory. In 1926, following Kamerlingh Onnes’ death, he compiled a catalogue of the historical collection. It was to form the starting point for the foundation in 1928 of what was then called the Netherlands Historical Museum of Natural Sciences.

Three years later, the museum opened its doors to the public. Right from the start, the collection also included items relating to medicine. Technology was welcome on condition it served fundamental research; such technical applications as an alternator were beyond the scope of the collection, as were domestic appliances. Crommelin travelled to all corners of the country to expand his collection. In 1947, the private Historical Museum of Natural Sciences was closed, and the Dutch Government formally took over its running. The new name reflected its ambitions: National Museum for the History of Sciences. A committee of advisors comprised of professors from all the universities underlined the fact that this was a national museum.
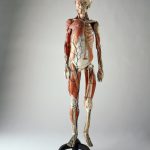
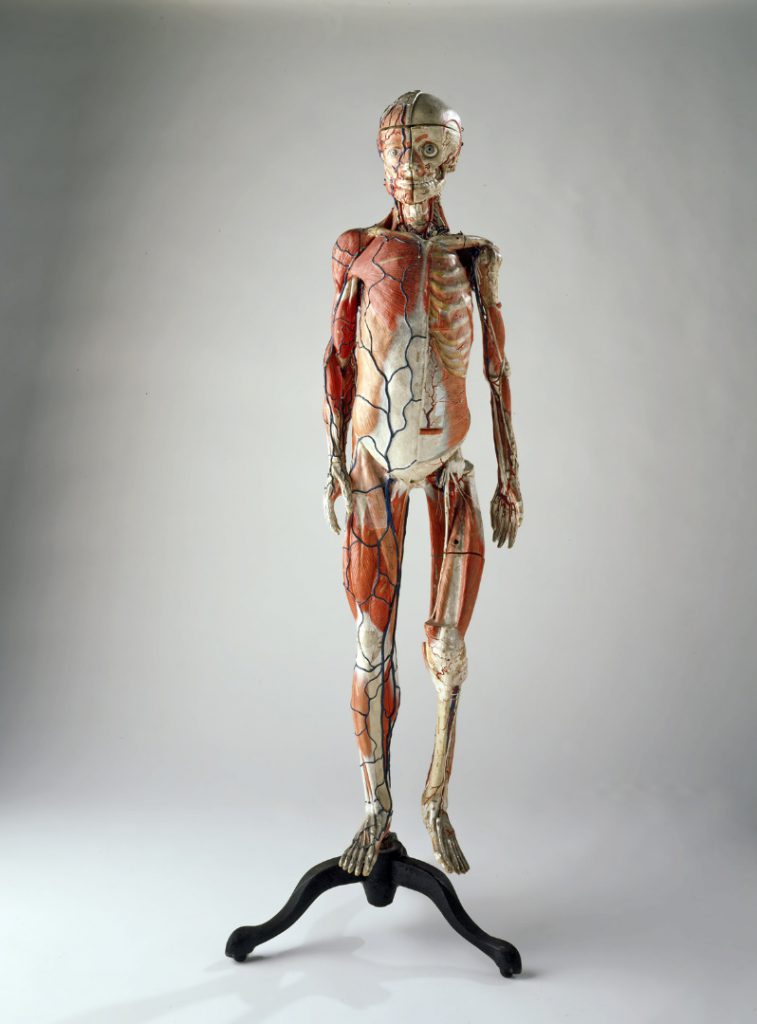
Nonetheless, Leiden is heavily represented in the collection. Among the museum’s most important pieces are the quadrant belonging to Snellius, the medical instruments of Solingen, the oldest herbarium in the Netherlands, a six metre-long extendable telescope by Huygens, the electrocardiograph with which Einthoven first recorded heart tones, the artificial kidney by Kolff, the collection of anatomical papier mâché models by Auzoux (see Figure 2), the meridian viewer from the Leiden Observatory and the Ploem’s epi illuminator (a breakthrough in fluorescent microscopy) (Delft, 2007). The name ‘Rijksmuseum Boerhaave’ dates from 1976. The current location, somewhat hidden away in the very heart of Leiden, became home to the museum in 1990. The move represented a huge step forward in terms of floor space for the presentation of the permanent and temporary exhibitions. At present, the collection totals some 40,000 objects (95 per cent are kept in store), excluding the books, prints and archives. The museum employs around 35 full-time staff.
It is important to understand that Rijksmuseum Boerhaave was established for a completely different reason than, for examples, the Science Museum in London or the Deutsches Museum in Munich. Whereas in the establishment of the latter two museums the ideas of the Age of Reason played an important role, namely to enlighten and educate the general public by exhibiting the wonders of science and technology, the main reason for the establishment of Rijksmuseum Boerhaave was to preserve from destruction the museum’s valuable collection (Bennett, 2006). Visitors were welcome (as long as they first rang the doorbell), but Crommelin was never truly the perfect host.
The Dutch Historical Museum for Natural Sciences was aimed more at the educated elite, insiders capable of appreciating the intrinsic values of these scientific and medical instruments, without requiring further explanation. Museum director Crommelin was not against the idea of welcoming ‘holidaymakers’ who on a rainy day paid the museum a visit when there was nothing else to do, but his museum was above all the realm of a ‘more enlightened public’.
This spirit of Crommelin was long upheld by the museum. When the author of this article became director in 2006, the main focus remained on the collection, while the public came in a clear second. Take for example the permanent exhibition at Rijksmuseum Boerhaave. The concept behind this exhibition dates from the late 1980s when the museum moved to its current location, the Caecilia Gasthuis (a former hospital). The permanent presentation, a chronological tour through 24 rooms, is hallmarked by its emphasis on aesthetics and the considerable focus on the object (see Figure 3). Indeed, it could be characterised as an altar to the scientific instrument. From clean-cut glass cases the observer is, as it were, ‘granted’ a view of these unapproachable instruments. In many places there is no clear context and the choice of object reflects no semblance of any hierarchy.
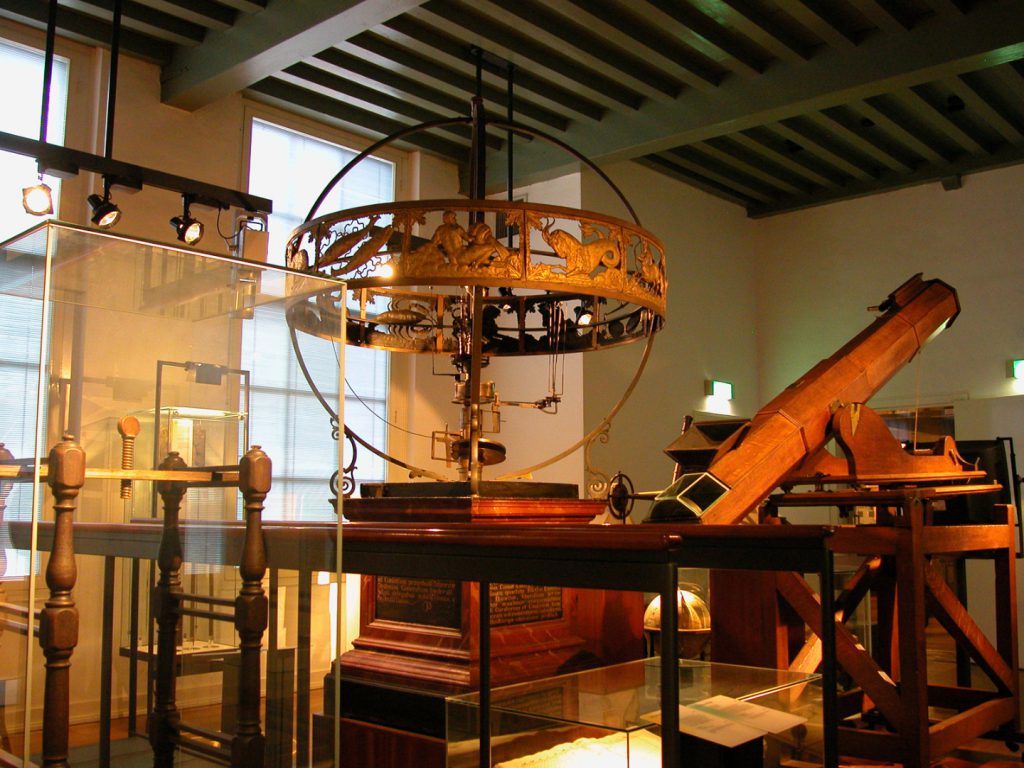
Any visitor who lacks scientific or medical training rapidly feels overwhelmed and very much left to their own devices in this atmosphere so clearly intended for scholars. It is up to the individual to find his or her own way around the exhibits, and for children the experience is less than enchanting. Anyone taking in the permanent presentation in the accompaniment of an expert guide will gain much insight from the explanations. A visitor with no such guide, who as a result misses the accompanying stories and explanations of the complex instruments, soon becomes intimidated or engulfed by the apparent surplus (‘yet another glass case containing microscopes’). Even the very best audio tour is not enough to brighten up such an exhibition. The biggest problem is that the people who actually worked with the instruments in practice are absent, and no attention is paid to the era in which they lived. It is for these reasons that over the coming years, Rijksmuseum Boerhaave intends to thoroughly overhaul its permanent presentation. In essence, the intention is less brass and glass, more flesh and blood and stories (Maas, 2010).
An essential starting point for the new permanent presentation is a redefinition of the target groups for the museum. First of all, Rijksmuseum Boerhaave is more than an (inaccessible) mecca for connoisseurs of scientific instruments. The challenge lies in also making the museum attractive to culture-loving Dutch (and foreign) visitors, from the point of view of the vision that the natural sciences and medicines are not context-less activities, but form an integral and thoroughly embedded part of our culture. Numbers of both individual visitors and group visits (above all schoolchildren) can be considerably raised by increasing the attractiveness of the museum through a series of targeted interventions. As well as a new permanent presentation, these activities will include launching a ‘Rijksmuseum Boerhaave Junior’, laying out an educational water playground in the walled garden and extending the educational workplace Technolab, which provides classically-taught workshops in technical studies to schoolchildren between the ages of 4 and 14 years. This combination will result in an appealing range of educational facilities and will increase the attraction of the museum to families with children. None of these measures, however, mean that the museum will be prostrating itself to mere fun. Rijksmuseum Boerhaave will continue to focus fully on an audience wishing to learn something. People of all ages continue to demonstrate curiosity and a keenness to learn. Rijksmuseum Boerhaave does not intend to provide pretention-free enjoyment, but instead wishes to take up the challenge of placing sometimes difficult subject matter in the spotlight, in an attractive, accessible and playful manner.
All of these objectives will have consequences for the permanent presentation. Any vital permanent presentation is permanently in flux. The most important change will be that Rijksmuseum Boerhaave will no longer be charting out five centuries of innovation in the fields of physics and medicine through what is (sometimes) a surfeit of interesting and/or aesthetically-pleasing instruments. Instead, the museum will tell a tale of primarily cultural history, whereby the history of science will be given a place in a broader sociocultural context – wherever possible with references to the present day. This story will be backed up by a careful selection of objects (along the lines of less is more) in order to provide sufficient space for laying out the relevant context. Further support will take the form of short films, photographs, animations and interactive ICT solutions (smart audio/video tour on PDAs or iPads), hands-on games, models, an attractive design and other more theatrical means of creating experience and action. In the new permanent presentation, the central figure will be man, while a story will be told that will be of interest to both young and old. A start has already been made on the development of the overall story.
Both halls on the first floor of the museum containing the Leiden Physics Cabinet were redesigned on the basis of the principles outlined above (see Figure 4). The aim was to experiment with content, layout, storyline and design to inform future exhibitions, guided in part by lessons learned during the development of the earlier NewtonMania exhibition (December 2009–September 2010). The project allowed the museum to investigate the response from the public while encouraging enthusiasm by other stakeholders (for example the Ministry of Education Culture and Sciences, the cultural funders, sponsors, etc.). Following on from this exhibition, the museum has developed an extensive plan for the phased restructuring. This plan should result in a completely renewed Rijksmuseum Boerhaave, one that offers real meaning and which can face the future with confidence.

Liquid helium
https://dx.doi.org/10.15180/170812/003The temporary exhibitions at Rijksmuseum Boerhaave are also set to undergo a transformation. This development brings us back to Quest for Absolute Zero, the first major temporary exhibition established according to the new principles that on all fronts represents a break with the past. As already indicated, the background to the exhibition was ‘one hundred years of liquid helium’. Thanks to the loving care of August Crommelin, the cryogenic laboratory of Kamerlingh Onnes is well represented in the Rijksmuseum Boerhaave collection, and with a director who himself obtained his doctoral degree from the University of Leiden in 2005 on the basis of a scientific biography of Kamerlingh Onnes, the temptation to dedicate an exhibition to ‘10 July 1908’ proved irresistible.
But what kind of exhibition should it be? What story should the exhibition tell? To whom and how? What was the target group? How should the exhibition relate to the ‘cold objects’ already present in the permanent exhibition? What roles should fundamental research, the related technology and the people who undertook the work in practice play? What opportunities were available for collaboration with others, and how broad should the exhibition be?
To start with, the story of the liquefaction of helium, as recorded in the biography (Delft, 2007). The story goes as follows: On 10 July 1908, Heike Kamerlingh Onnes was the first to master helium. In room E of his Physical Laboratory, in the very heart of Leiden, after a long and exhausting day, he succeeded in liquefying the last of the ‘permanent’ gases. He thereby achieved a temperature just a few degrees above absolute zero (-273 °C) and Leiden became the coldest place on Earth.
The attack on helium was a carefully planned mega operation. Early in the morning, even before the birds were up, Kamerlingh Onnes had been driven to his laboratory by coach and horses from Huize ter Wetering, his country estate on the Galgewater. There, he immediately donned his white jacket and joined his technicians who had already been hard at work for hours producing liquid hydrogen that together with liquid air was to be used as the precooling agent for the helium. While the Burckhardt pumps squeaked and ground in room A, Kamerlingh Onnes and his workers conscientiously twiddled taps, disconnected gas bottles and anxiously monitored manometers and thermometers. There was no time to snatch even a single bite to eat. By one thirty in the afternoon, twenty litres of liquid hydrogen had been drained into thermos bottles (known as Dewar flasks), sufficient to begin the attack on helium.
At 4:20pm (Onnes’ wife Betsy had by this time anxiously popped by to check on progress, and to feed scraps of bread to her husband as he slogged away) the pump was switched on to allow the helium to circulate. The approach was to first compress the helium to 100 atmospheres, and subsequently to allow it to expand via a porous plug into a vacuum, thermally insulated by a complete ring of thermos flasks containing liquid air and liquid hydrogen. In this way, with every turn around the apparatus, the helium cooled slightly further and the eventual hope was that the fall in temperature would continue to the point at which the helium would condense.
Initially the experiment looked set to fail. One thermometer had already given up the ghost and the second almost refused to fall at all. To Onnes’ relief, however, the cooling process did begin, and at around 6:30pm the temperature was below that of liquid hydrogen. With fluctuating progress, the readout fell to just 6 degrees above absolute zero. By this time, although Kamerlingh Onnes had connected the final bottle from the store of liquid hydrogen to the apparatus, little more had been seen than the occasional swirl.
Suddenly, at -269 °C, four degrees above absolute zero, the thermometer started to indicate a remarkably stable value. This took place around 7:30pm. A colleague, who out of curiosity had come in to check on progress, suggested that there was already liquid present. Illumination by a further lamp indeed indicated that the thermometer was suspended in liquid helium. Contact wires could clearly be seen penetrating the surface of the liquid. ‘Once we actually saw the surface,’ Kamerlingh Onnes wrote in his report for the Academy of Sciences, ‘we never let it out of our sight. It remained clearly visible against the glass wall.’
The experiment had resulted in sixty millilitres of liquid helium, just about a teacup full. After undertaking a number of initial experiments with his new liquid – an attempt to actually freeze it by establishing a vacuum failed – Kamerlingh Onnes decided at 9:40pm that enough was enough. ‘Not only had the equipment been tested to its absolute limit in this experiment and in its preparation,’ he wrote in his Academy report, ‘the maximum possible had also been demanded of my assistants.’
The liquefaction of helium is a highpoint in Dutch (and international) physics. At the same time, it represented the culmination of a programme launched by Kamerlingh Onnes upon his appointment in Leiden in 1882, which he had implemented with a firm hand, a talent for organisation and tremendous perseverance. In his inaugural speech (in which he first coined the phrase ‘Through measurement to knowledge’), Kamerlingh Onnes had announced his wish of testing through experimentation the validity of the molecular theories of his colleague and friend Johannes Diderik van der Waals. The construction of a cryogenic laboratory, a cold factory of never before seen proportions, was the unavoidable consequence of that decision because ‘simple’ substances such as the biatomic O2 and H2, as well as noble gases like helium (discovered on Earth by William Ramsay in 1895) could only be condensed into liquid at extremely low temperatures.
Kamerlingh Onnes’ approach was based on two foundations: accuracy and cold. The first was necessary because the laws of van der Waals only applied by approximation, as a consequence of which the deviations from those laws were needed if physics was to be advanced. That process itself calls for precision measurements, something very close to the heart of Heike Kamerlingh Onnes. During the first period of Kamerlingh Onnes’ professorship, the establishment of a cryogenic laboratory demanded all his energy. Step by step he expanded his empire until it became a bustling cryogenic institution of global proportions; a laboratory offering unique opportunities for research. From all corners of the globe, guest researchers (and visitors) travelled to Leiden (see Figure 5). Kamerlingh Onnes did his level best to make his foreign guests feel welcome, regularly accommodating the more celebrated among them in his villa, Huize ter Wetering. His efforts enabled him to achieve his ideal, namely establishing an international reputation for Dutch physics.
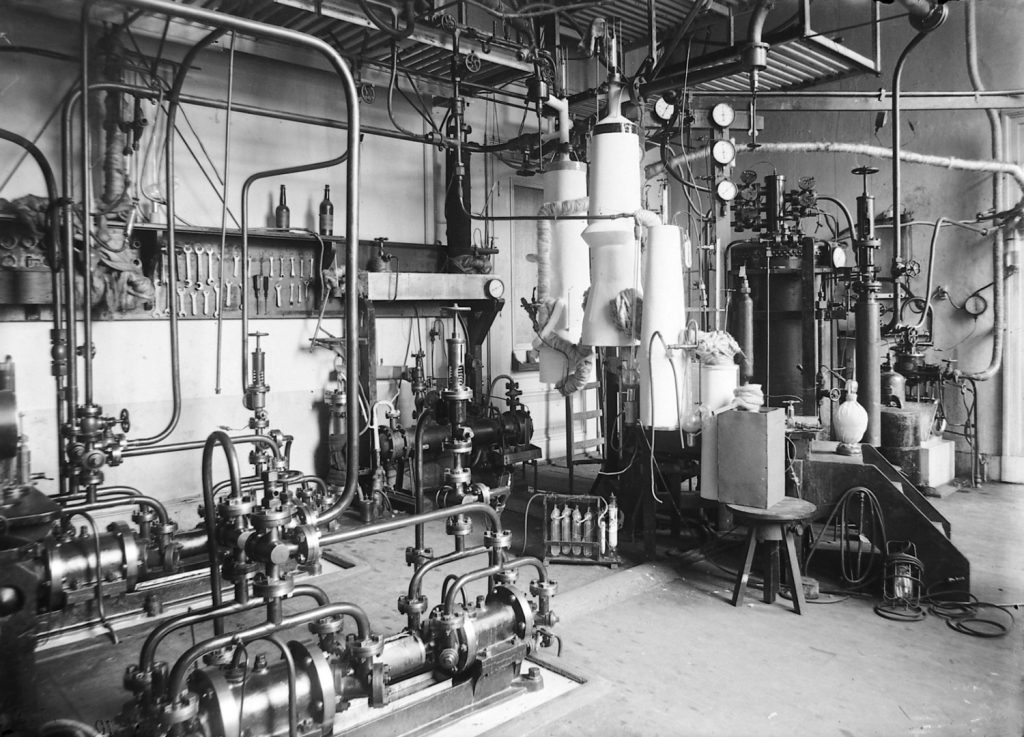
Kamerlingh Onnes invested all his energy in his laboratory and in the unique apprentice system for instrument makers (‘the blue boys’) that together formed an ‘organic unit’. Beyond his work, the only thing that mattered to him was his family. Huize ter Wetering was the meeting point for an artistic family, a place that exuded a cosmopolitan atmosphere partly thanks to the presence of innumerable foreign scholars who stayed there (Niels Bohr, Albert Einstein, Madame Curie, William Ramsay) and such visiting artists as Jan Toorop and Albert Verwey.
Heike Kamerlingh Onnes drove his people on like the wind drives the clouds. The true miracle is that a beanpole of a man who as a child spent a year ill at home reading Plutarch, and was forced every summer throughout his life to travel to the Alps as a treatment for his chronic bronchitis, nonetheless found the breath and the energy to establish and even to carry though to fruition an enterprise on the scale of a cryogenic laboratory. Assisted by his wife Betsy, Heike succeeded in focusing his limited strength on one goal: his cold laboratory. Following the success of liquid helium, he discovered superconductivity in 1911 and in 1913 won the Nobel Prize for Physics (for his helium work; the Nobel Committee never even mentioned superconductivity). At the end of his career, in 1924, he managed to bring in a donation of 100,000 guilders from the International Education Board of the Rockefeller Foundation, for the further expansion of his laboratory.
New approach
https://dx.doi.org/10.15180/170812/004How then should Rijksmuseum Boerhaave translate this success story about liquid helium and the man who turned Leiden into the ‘coldest place on Earth’ into an appealing exhibition for the general public? That this would be a major challenge was certain. At the start came the issue of the degree of difficulty. Anyone wishing to explain the helium apparatus is faced with a considerable challenge. Thermodynamics is not a subject that gives many people a warm, cosy feeling. Nonetheless, in an exhibition about achieving temperatures close to absolute zero, it is difficult to avoid mentioning the underlying principles. But just how far should you go? For a specialist, the Joule-Thomson effect – the cooling of a gas as it is forced through a porous plug and subsequently expanded – is essential in producing liquid helium. For the uninitiated, however, the interest is minimal, and the question was to what extent he or she would be willing to make any effort to, for example, understand the functioning of a refrigerator.
Then there was the problem of the low ‘bling’ value of the object in questions. While brass proportional compasses from the Dutch Golden Century or an eighteenth-century vacuum pump by Jan van Musschenbroek from the Leiden Physics Cabinet are beautiful to behold, even if you do not understand their operation, the aesthetic appeal of an ethylene boiling bottle or a Cailletet pump is limited (see Figure 6) (de Clercq, 1997).
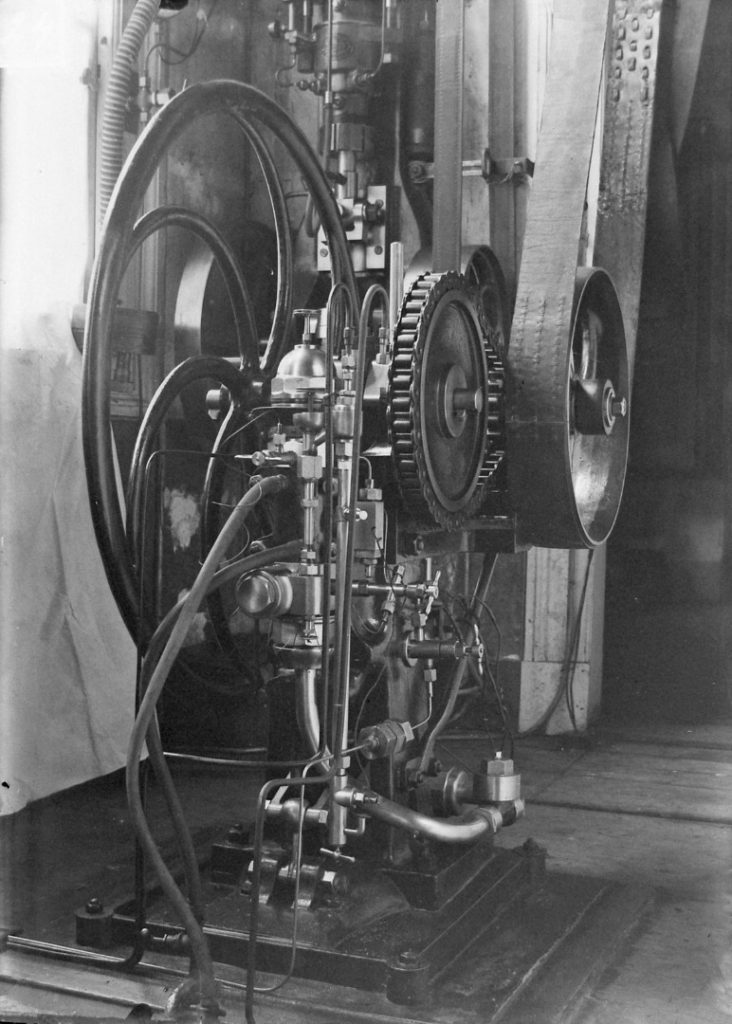
Their very coarseness creates a sense of distance. Ask a curator at Rijksmuseum Boerhaave to identify objects that appeal to a broad public and he (or she) will undoubtedly rummage around in the medical collection, preferably for something to do with infectious diseases that lead to malformation of the face – shudder as you enjoy the horror. In that respect, the collection of the Leiden cold laboratory has little to offer. Colossal compression and suction pumps made by the Societé Génevoise in Basel or the 1908 Leiden helium liquefier may be enough to take technicians and physicists to seventh heaven, but such specialists are limited in number and the general public has no real interest in difficult-to-understand devices and instruments that exude nothing but a taste of things technical from their glass cases or platforms. The general public is only ready to accept into their heart these difficult objects if they form part of an accessible and attractive cultural or historical story.
On top of that, the main character in the exhibition, Heike Kamerlingh Onnes himself was not even a colourful figure. The ‘absolute zero man’ was a hard worker who operated with diplomacy and outside his laboratory led a relatively invisible life. The 64 million-dollar question for the exhibition makers at Rijksmuseum Boerhaave was who would possibly wish to identify with a bald-headed boffin?
To tackle all of these problems, the decision was taken to base the exhibition around the theme of ‘Rivalry in science’. The liquefaction of helium is not presented as a technical feat of arms worthy of respect, but as the apotheosis of an international struggle. The starting point was that the quest for absolute zero is an exciting story peopled by colourful characters. Tragedy, heroism, disappointment, joy, setback, argument and eventually the victory by Kamerlingh Onnes on 10 July 1908 – all are featured. The human scale and the colourful story behind the man and his pump had to be the centrepiece, rather than the scientific background or the technical solutions. The choice was to focus on Kamerlingh Onnes’ rivalry with Dewar in London, setting up a competitive story that is very familiar in public accounts of science. From the perspective of the historian of science such a ‘one dimensional’ approach is problematic. On the other hand, rivalry is part of science and focusing an exhibition on this aspect has its own merits. To tackle the rivalry simplification, the exhibition was accompanied by the book Quest for Absolute Zero, written for the occasion by Dirk van Delft, expert in the history of low temperature physics.
The choice of exhibition designer proved important in this connection. The layout had to break with the clean-cut, aesthetic form of the traditional exhibitions at Rijksmuseum Boerhaave, for which the same agency – The Hague-based 2D3D – was always awarded the contract, and always with the same budget. The new chosen agency was Meulendijks Art Direction based in Rotterdam. They came up with a playful, theatrical approach that almost magically transformed the two Boerhaave galleries of the museum (the location for all temporary exhibitions) into an appealing ‘theatre of cold’. In financial terms, too, Meulendijks was given more freedom. The exhibition budget was more than twice the amount usually spent in the past. The museum was able to allow itself this luxury, having attracted external funding.
For the first time in its history, Rijksmuseum Boerhaave had actively – and successfully – gone in search of sponsors in the run-up to the Quest for Absolute Zero. The cultural funds, representatives of business and industry and the Dutch scientific community dug deep. The funds appreciated the cultural historical approach and the involvement of external parties in the project, in particular the Lakenhal City Museum and the Law Faculty of the University of Leiden (which since 2005 has been headquartered in Kamerlingh Onnes Laboratory). Linde Gas, international suppliers of helium and cryogenic equipment, came forward as main sponsors. The company is named after Carl von Linde, the German cold pioneer who at the end of the nineteenth century established a refrigerator factory in Munich, thereby taking the first step in creating what has today become a world-encompassing empire. In the exhibition, Carl von Linde was present in the ‘hall of fame’ of low temperature scientists (in the Quest for Absolute Zero book the von Linde company was presented in the chapter on applications). Government institutions also took part: NWO (the Netherlands Organisation for Scientific Research) and the Bèta technology platform chipped in, as did ‘physics’ foundations such as the Lorentzfonds, the Pieter Zeemanstichting, the Gorter Fonds and the Stichting Physica. Liquid helium, it soon became clear, was assured a warm welcome.
In the new design the content of the exhibition tied in closely with its form. The quest for absolute zero was broken down into a series of episodes, each of which featured rivalry, heroism, tragedy and triumph. The story started with the liquefaction of oxygen (1877; Karol Olszewski and Sigmunt Wroblewski versus Raoul Pictet), followed by liquid hydrogen (1898) and liquid helium (1908; James Dewar versus Heike Kamerlingh Onnes), after which the struggle still went on taking the visitor via Bose-Einstein condensation (1996; Wolfgang Ketterle versus Carl Wiemann) to today’s leading edge of cold research. Today, absolute zero has been reached to within one billionth of a degree. The challenge lay in avoiding a series of unconnected feature pieces, as is normally the case in the permanent presentation of Rijksmuseum Boerhaave, but instead selecting objects for their contribution to the overall story, and placing them in a refined manner in a context, thereby melding them into a single coherent unit. The theatrical background from the exhibition’s designers almost automatically resulted in an evocative and accessible design that enabled visitors to empathise with the story. It appeared as if the main characters from the Quest for Absolute Zero had momentarily left their experimental laboratories, to allow the visitor a peek behind the scenes.

It is an objective of Rijksmuseum Boerhaave, even in its temporary exhibitions, to start with pieces from its own collection, wherever necessary supplemented by loaned exhibits from third parties. After all, the museum itself started with the goal of maintaining the real thing. For this exhibition a number of objects (pumps, electrical resistors, cold bottles) were transferred from the permanent presentation. The fragile helium liquefier, the absolute jewel in the crown, was not included due to the risk of fracturing the glass if it were moved. Instead, visitors to the Quest for Absolute Zero were presented with a photograph and the firm recommendation to make a point of visiting the original downstairs. From the museum’s store room came Kamerlingh Onnes’ own massive roll-top desk, barometers standing metres tall, and thermometers. Most astonishing, in terms of size at least, a cryostat recently removed from the measuring gallery of the new Kamerlingh Onnes Laboratory was transferred to Rijksmuseum Boerhaave with all the accompanying instruments for the millikelvin range. Original observation books and other archive documents and personal effects completed the pieces on display.
No exhibition could be complete without loaned pieces. Among the most important were a Dewar flask dated 1894 (a twin-walled vacuum thermos flask, produced by James Dewar in the Royal Institution), and the glass tubes in which William Ramsay (in 1895) sent the helium gas he had just discovered to Cracow rather than to the Royal Institution because of a dispute with Dewar. Another significant loan was a severely-damaged manometer from Cracow, the result of an explosion in Olszewki’s laboratory in that same city (Wroblewski, from whom Olszewki was estranged shortly after their success with liquid oxygen, died from his injuries, following an accidental fire). High drama indeed!
To make the exhibition more attractive, and to convincingly present in all its glory the underlying story of the rivalry between researchers – every one of whom wanted to be first – no stone was left unturned. To clarify the basic principles of the underlying technology, students of the Rotterdam art academy produced easily digestible animations, and in the exhibition itself, low-threshold demonstration rigs were installed, that could easily be operated by the visitors. To create a laboratory atmosphere, in addition to the original pumps and cold bottles, life-size blow-ups of original photographs were featured. A cardboard Dewar and Kamerlingh Onnes debated with one another via projected text bubbles and a radio play was even produced of Kamerlingh Onnes’ eyewitness report of the liquefaction of helium on 10 July 1908, published by the KNAW (Royal Netherlands Academy of Sciences). Use was also made of historical film fragments and key scenes from the TV documentary Absolute Zero (2008) by the British director and producer David Dugan. In that documentary, actors replayed the triumph of 1908 against the background of the second-generation helium liquefier, erected in the Leiden Oortgebouw, accompanied by ancient pumps and magnets. The scene in which Mrs Kamerlingh Onnes feeds scraps of bread to her hard-at-work husband featured prominently. Specially for children, at various point throughout the exhibition, mini refrigerators were placed, each containing an assignment, for which prizes could be won on completion. At various points in the story, modern applications were displayed. Close to the Dewar flasks, for example, modern thermos flasks were featured, while the early-twentieth-century refrigerator from General Electric was accompanied by its ultramodern counterpart.
Didn’t all this attention to context, to image, to sound and to moving elements distract from the real objects? On the contrary. Take the helium liquefier. If this were simply an isolated object on an altar, you could write on its showcase text that it is the instrument in which helium was liquefied for the first time at a record temperature of four degrees above absolute zero. However, if the same liquefier is displayed as the climax of an international struggle that lasted decades, there is so much more to say. In that case, the visitor could learn of Kamerlingh Onnes’ many years of hard work, his perseverance and his ‘big science’ approach. By telling these stories, the visitor’s awareness of the historical significance of the helium liquefier can only be enhanced. The one thing you have to make sure of is to not let the object be overpowered by too glaring a design.
To summarise, an attractive exhibition starts with a good story. A story that’s captivating for the visitor, a story that depicts science as a human endeavour peopled with characters of flesh and blood, afflicted with their qualities and weaknesses. A story with a message that goes beyond the topic that is being presented, a story that communicates to the public what science is about in a more general sense, a story that deals with the position of the scientist as a human being. A story that seeks connections between the topic of the exhibition and the contemporary world of the visitor.
In the case of Quest for Absolute Zero this approach led to a process of selecting artefacts on the basis of their potential to contribute to the story. The objects in the exhibition had to trigger the visitor’s empathy. Aesthetics or the iconic status of an object are less important in such an approach. A distorted gauge from the Polish laboratory of Karl Olszewski, the consequence of an explosion, shows like no other masterpiece that the quest for absolute zero was not without risks – risks the scientists involved were willing to take. And Cryo-1, the freshly acquired low temperature machinery from the Kamerlingh Onnes Laboratory, shows in its shiny complexity and overwhelming dimensions how the quest for absolute zero has changed. No longer a romantic enterprise with scientists working on their own as they did at the turn of the last century, it now involves large scale industrial efforts requiring lots of space, lots of technicians and lots of money.
An important factor in the success of Quest for Absolute Zero was the collaboration with the Lakenhal art museum, just a few minutes’ walk from Rijksmuseum Boerhaave. The Kamerlingh Onnes family excelled in producing cold, but also in producing works of art. Heike’s brother Menso and Menso’s children Harm and Jenny together produced a huge number of paintings, watercolours and pencil drawings in which they also featured many of the people and objects relating to the cold laboratory (see Figure 8). Heike supported these activities and encouraged his nephew Harm to visit his laboratory, and to bring his sketchbook with him. In 1920, he also asked Harm to design stained-glass windows on the occasion of the 25th anniversary of the Zeeman effect, nicely depicting the Nobel Prize-winning discovery by Pieter Zeeman and its theoretical explanation by Hendrik Lorentz. In order to bring together both worlds, a Lakenhal art museum exhibition entitled The Kamerlingh Onnes Family: Cold & Art ran parallel to the exhibition at the Rijksmuseum Boerhaave. By working with a single entrance ticket to both exhibitions, a sort of cross-pollination occurred between the two different types of visiting public, encouraged by the proximity of both locations. This combination was much appreciated.

Also, elsewhere in Leiden, there was interest in the ‘absolute zero man’. There were minor exhibitions about Kamerlingh Onnes in the Leiden University Library (books, letters and magazines), in the Leiden Municipal Library (featuring the same sort of material) and in the Kamerlingh Onnes Building, where photographs of the former laboratory were exhibited together with a series of sketches by Harm Kamerlingh Onnes. As extras, we organised a bicycle tour of historical locations related to science; in the framework of an educational programme, schools could book a combined ticket to visit the exhibition and the Freezing Physics show, provided by physics students.
Conclusion
https://dx.doi.org/10.15180/170812/005In the Quest for Absolute Zero, Rijksmuseum Boerhaave made both the content of cold science and the action of applied cold technology subservient to the underlying human story; a story with glorious victors and tragic losers, with brilliant inspirations, incredible perseverance, tunnel vision, force of will, camaraderie, argument – much argument – and exploding apparatus. We were celebrating a Nobel Prize and commemorating a death. A relatively new historical collection of inaccessible and relatively unattractive objects with no intrinsic eloquence was brought to life by placing it in a context of a scientific race – featuring elements of theatrical backdrop, film fragments, photographic blow-ups, animations and games – and by the powerful storyline.
The success of this approach, given the scale of the external financial contributions, the massive rise in visitor numbers during the course of the exhibition and the appreciation expressed by those visitors (also for the combination with the Lakenhal exhibition) proved a real boost for the plans of Rijksmuseum Boerhaave to redevelop its permanent exhibition along similar lines. From a museum merely housing objects, Rijksmuseum Boerhaave wishes to make the transition to a story-based museum, still featuring the original objects but viewed from the point of view of cultural history rather than instrument history. After all, colourful stories about science and scientists are capable of generating enthusiasm and understanding for the exact sciences, perhaps more effectively than the sometimes overly weighty educational approach employed by certain fellow museums.
To conclude, the balance of the Quest for Absolute Zero project was extremely successful. It took a great deal of work, and at the time the Rijksmuseum Boerhaave organisation was not used to large and sometimes complicated projects of this nature. Nonetheless, we succeeded, we learned a great deal and we had plenty of fun. The exhibition was featured on television news; we attracted numerous extra visitors and, an important element for the future, we enjoyed a boost to our reputation among our colleagues, the funding agencies, the academic world and, last but not least, our visiting public.
Tags
Footnotes
Back to text